Serine Protease Inhibitors Mechanism Of Action
Isocoumarins are potent mechanism-based heterocyclic irreversible inhibitors for a variety of serine proteases. Serine protease inhibitors have also been used to treat atopic dermatitis using α1antitrypsin inhibitor with the authors at the time fortuitously interpreting that atopic dermatitis may be one example where inflammation is due to an imbalance of serine proteases and their naturally occurring inhibitors.

Mechanism Of Inhibition Of Serine Proteases By Download Scientific Diagram
Chymotrypsin trypsin and elastase.

Serine protease inhibitors mechanism of action. Serine proteases and their natural protein inhibitors are among the most intensively studied protein complexes. The most useful types of inhibitors for serine proteases are transition-state inhibitors including alpha-ketoesters and phosphonates and mechanism-based inhibitors such as heterocyclic isocoumarin. The canonical inhibitors bind to the enzyme through an exposed convex binding loop which is complementary to the active site of the enzyme.
The hemostatic mechanism of action of aprotinin. Mechanism-based isocoumarin inhibitors for serine proteases. The first synthetic serine protease inhibitors to be extensively explored were tripeptides such as 1 6.
Trap mechanism of action in which a proteinase binds to a generic bait. Aspartic proteinase inhibitors Pepstatin Cysteine proteinase inhibitors Cystatin Metalloproteinase inhibitors Tissue inhibitors of metallopro-teinases Serine proteinase inhibitors Alpha1-proteinase inhibitor Antithrombin III Fig 1. About 20 structurally diverse inhibitor families have been identified comprising.
We will look at the enzyme mechanism of chymotrypsin in detail. In the present study we compared chloro- and nitrile-substituted peptidyl 14-naphthoquinones with respect to their inhibition properties. The non-canonical inhibitors interact through their N-terminal segment.
The purpose of this review is to discuss the probable mechanisms of aprotinin action from the perspective of its interactions within the hemostatic and. However remains to be elucidated fully. The serine protease inhibitors comprise a large family of molecules involved in inflammatory responses blood clotting and complement activation.
Journal of Cellular Biochemistry 1989 39 1 33-46. Alpha-1 proteinase inhibitor is a serine protease inhibitor Serpin. The hemostatic mechanism of action of aprotinin.
These compounds are referred to as reversible transition-state inhibitors RTSI because they contain a C-terminal group which binds in a reversible fashion to the serine hydroxyl group at the enzyme active-site and mimics the transition-state achieved when the enzyme cleaves the substrate. Use of active site structure and substrate specificity in inhibitor design. Nitriles constitute another important substance class of protease inhibitors which inhibit cysteine and serine proteases in a covalent reversible manner via the formation of a thio imidate 1821.
Its primary mechanism is inhibiting the action of the serine protease called elastase also plasmin and thrombin in the lungs. Alpha2-macroglobulin mechanism of action. Prostasin gene expression is down-regulated in high-grade and hormone-refractory prostate cancers.
Standard mechanism of protein serine protease inhibitors bind in a substrate-like manner that completely spans the active site and act as substrates with a very slow kcat. Two candidate sites were localized to regions AA1-273 and AA273-410. Family of natural inhibitors called serpins can form a covalent bond with serine protease.
There are three well known enzymes that go through the serine protease mechanism of action they are. A protease is an enzyme that hydrolyzes peptide bonds that link amino acids together in a protein. The reactive center loop RCL of alpha-1 proteinase inhibitor extends out from the body of the protein and directs binding to the target protease.
In particular the serine protease inhibitor aprotinin consistently reduces post-operative bleeding. They interact with both the substrate binding sites shallow indentation and the catalytic residues rectangle of the serine protease. In the normal animal both inhibitors are constitutively expressed.
The purpose of this review is to discuss the probable mechanisms of aprotinin action from the perspective of its interactions within the hemostatic and inflammatory pathways. In particular the serine protease inhibitor aprotinin consistently reduces post-operative bleeding. Most serine proteases are inhibited by the general serine protease.
Serpins are known for their unusual mechanism of action. Unlike the common competitive mechanism of protease inhibitors that bind to protease active sites and block their entry serpins irreversibly inhibit the function of their protease by undergoing large conformational changes to disrupt the active site of it. However remains to be elucidated fully.
Serine proteases are inhibited by a diverse group of inhibitors including synthetic chemical inhibitors for research or therapeutic purposes and also natural proteinaceous inhibitors. The mechanism of inhibition in this group is always very similar and resembles that of an ideal substrate. The gene expression of two of the serine protease inhibitors SPI 21 and 22 is tightly controlled by growth hormone in rat liver.
These data support a mechanism of action for the matriptase-prostasin epithelial extracellular serine protease activation cascade by proteolytically modulating the EGF-EGFR signaling.
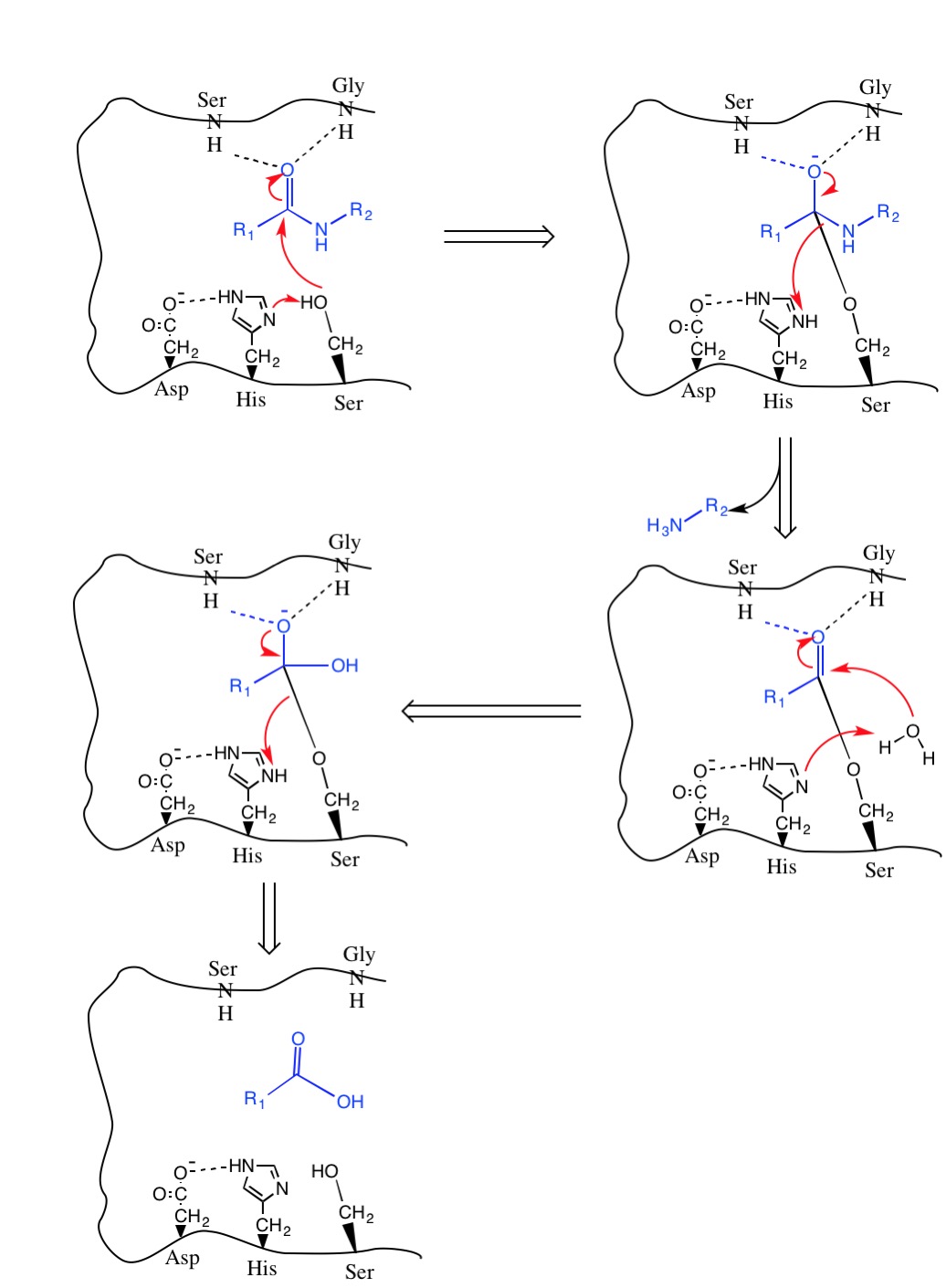
D Peptide Protease Resistance D Peptide Therapeutics

Microbial Serine Protease Inhibitors And Their Therapeutic Applications Sciencedirect

Mechanism Of Serine Proteases Inhibition By 1 Aminoalkylphosphonate Download Scientific Diagram

Mechanism Of Serine Proteases Inhibition By A Aminoalkylphosphonate Download Scientific Diagram

A Critical Review On Serine Protease Key Immune Manipulator And Pathology Mediator Allergologia Et Immunopathologia
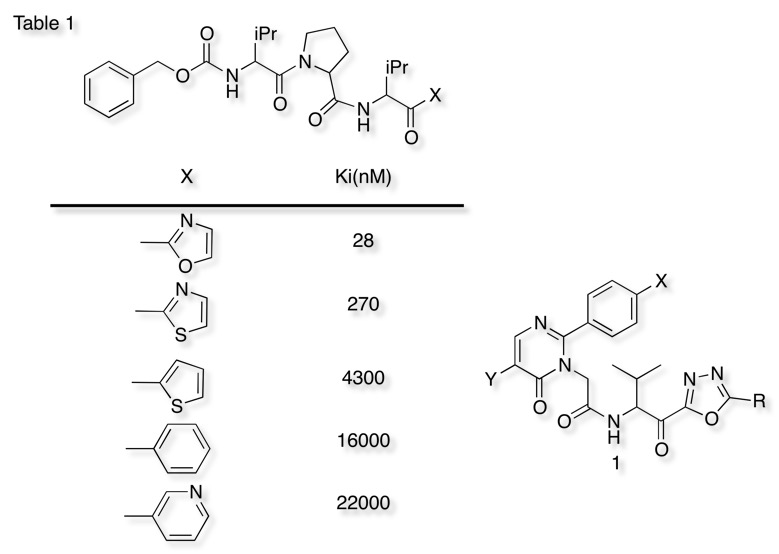
Serine Protease Inhibitors Cambridge Medchem Consulting

Enzyme Mechanisms Serine Proteases Youtube

Chemical Mechanism Of Catalysis For Serine Proteases Catalytic Groups Download Scientific Diagram

Mechanisms Of Protease Inhibitor Interactions A Irreversible Download Scientific Diagram

Serine Protease Inhibitors An Overview Sciencedirect Topics

Mechanism Of Serine Proteases Inhibition By A Aminoalkylphosphonate Download Scientific Diagram
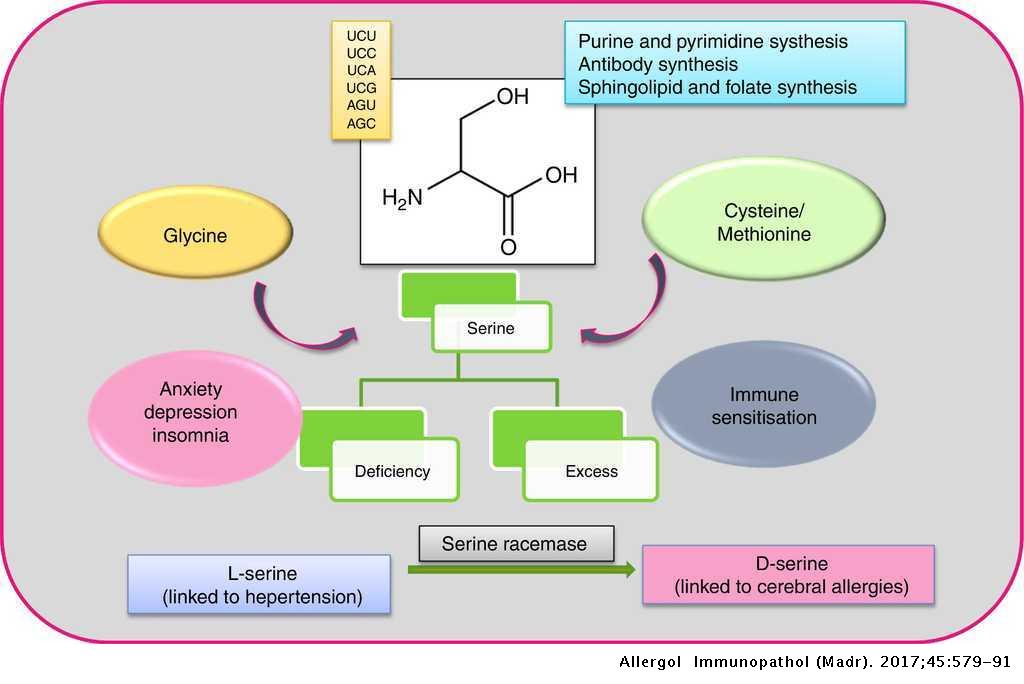
A Critical Review On Serine Protease Key Immune Manipulator And Pathology Mediator Allergologia Et Immunopathologia
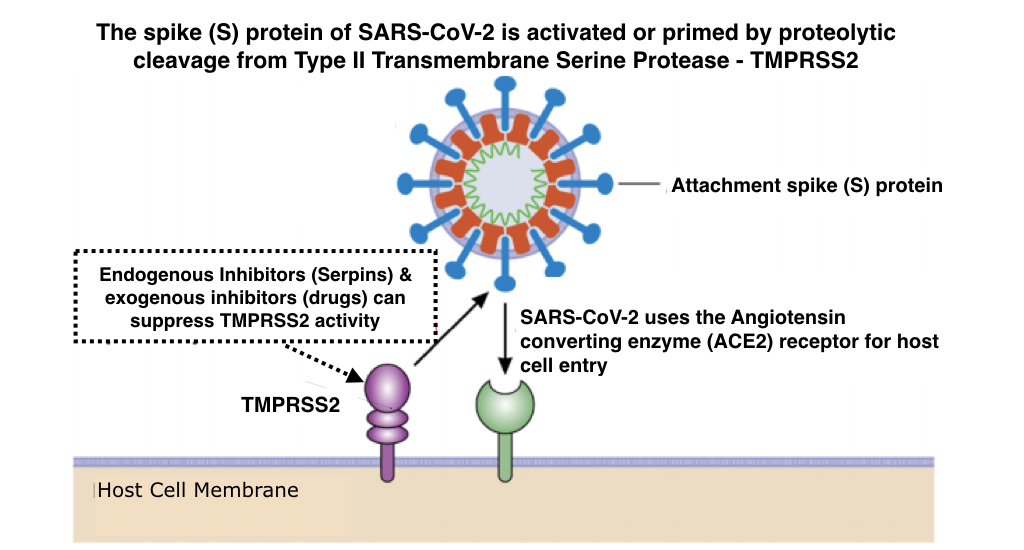
Patent Application Describes New Proteomic Methods
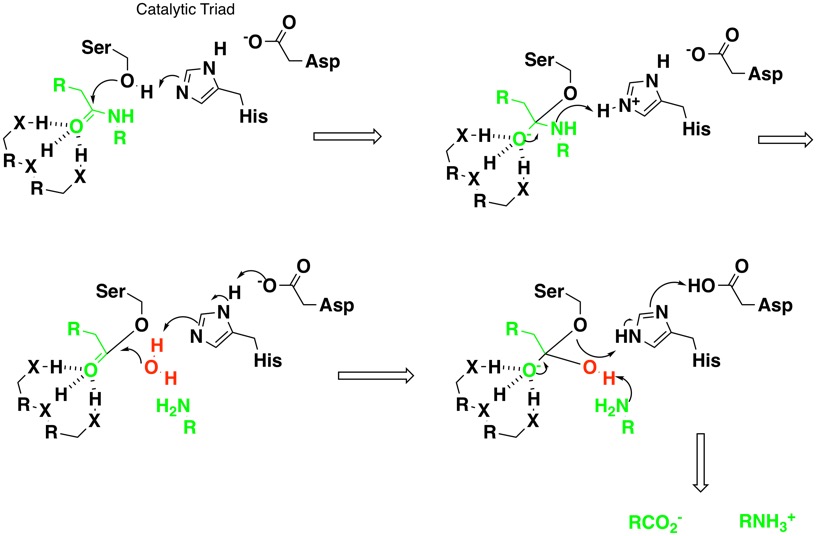
Serine Protease Inhibitors Cambridge Medchem Consulting

Serpin Structure And Mechanism Of Inhibition A The Rcl Blue Is Download Scientific Diagram
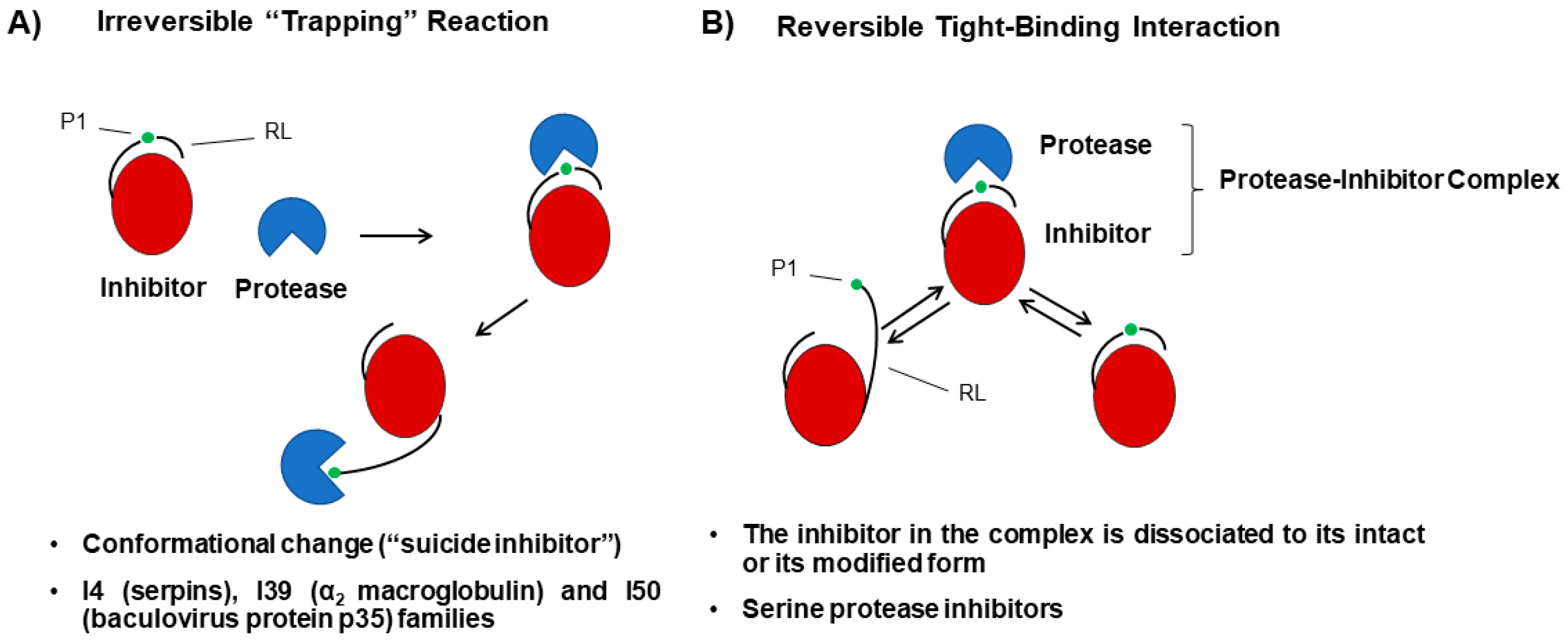
Ijms Free Full Text Plant Serine Protease Inhibitors Biotechnology Application In Agriculture And Molecular Farming Html

Disulfide Engineering Of Human Kunitz Type Serine Protease Inhibitors Enhances Proteolytic Stability And Target Affinity Toward Mesotrypsin Journal Of Biological Chemistry

Serine Protease Inhibitors An Overview Sciencedirect Topics

Mechanism Of Inhibition Of Serine Proteases By Download Scientific Diagram
0 Response to "Serine Protease Inhibitors Mechanism Of Action"
Post a Comment